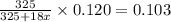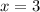Chemistry, 26.02.2020 18:29 unknown337
4. A 120.0 mg sample of a lead acetate hydrate, Pb(C2H3O2)2 × xH2O was heated to drive off the waters of hydration. The cooled residue had a mass of 103.0 mg. Calculate the value of x in the chemical formula. Show your work with Equation Editor.

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 07:30
What is i fracture in the crust called when land move up, down or sideways
Answers: 2

Chemistry, 22.06.2019 14:00
Displacement is the slope of a velocity vs. time graph a. true b. false
Answers: 1

Chemistry, 22.06.2019 16:00
Which of the following is the correct definition of chemical energy? a. energy an object has because of its motion or position b. energy resulting from the flow of charged particles, such as electrons or ions c. energy produced from the splitting of atoms d. energy stored in chemical bonds of molecules
Answers: 1

Chemistry, 22.06.2019 21:30
An atomic nucleus is composed ofa)protons.b)protons and neutrons.c)protons and electrons.d)protons, neutrons, and electrons.
Answers: 1
You know the right answer?
4. A 120.0 mg sample of a lead acetate hydrate, Pb(C2H3O2)2 × xH2O was heated to drive off the wate...
Questions


Mathematics, 19.10.2020 09:01


World Languages, 19.10.2020 09:01

English, 19.10.2020 09:01

Physics, 19.10.2020 09:01


Mathematics, 19.10.2020 09:01


Biology, 19.10.2020 09:01

Mathematics, 19.10.2020 09:01


Chemistry, 19.10.2020 09:01

English, 19.10.2020 09:01


Business, 19.10.2020 09:01


Biology, 19.10.2020 09:01


English, 19.10.2020 09:01

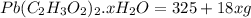
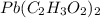 = 325 g
= 325 g
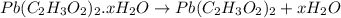
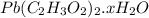 on heating gives = 325 g of
on heating gives = 325 g of 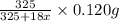 of
of