
Chemistry, 26.02.2020 17:47 clairebear66
Given the following balanced equation, determine the rate of reaction with respect to [O2]. If the rate of formation of O2 is 7.78 x 10-1 M/s, what is the rate of the loss of O3? 2 O3(g) → 3 O2(g)

Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 20:30
10. translate each of the following chemical equations into a sentence. a. 2 zns(s) + 3 o2(g) -> 2 zno(s) + 2 so2(g) b. cah2(s) + 2 h2o(l) -> ca(oh)2 (aq) + 2 h2(g)
Answers: 2



Chemistry, 22.06.2019 12:30
Suppose you wanted to make 100 grams of water. what is the molar mass of water (h2o)?
Answers: 2
You know the right answer?
Given the following balanced equation, determine the rate of reaction with respect to [O2]. If the r...
Questions


Mathematics, 02.06.2021 19:40


Spanish, 02.06.2021 19:40




Computers and Technology, 02.06.2021 19:40

History, 02.06.2021 19:40


Geography, 02.06.2021 19:40

Mathematics, 02.06.2021 19:40

Social Studies, 02.06.2021 19:40



Mathematics, 02.06.2021 19:40

Health, 02.06.2021 19:40

Mathematics, 02.06.2021 19:40

Mathematics, 02.06.2021 19:40

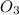 is 0.52M/s
is 0.52M/s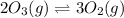
![-\frac{1d[O_3]}{2dt}](/tpl/images/0525/0164/0b459.png)
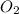 =
=![+\frac{1d[O_2]}{3dt}](/tpl/images/0525/0164/4dcb2.png)
![-\frac{1d[O_3]}{2dt}=+\frac{1d[O_2]}{3dt}](/tpl/images/0525/0164/b3aa3.png)
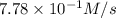
![\frac{2d[O_2]}{3dt}=\frac{2}{3}\times 7.78\times 10^{-1}M/s=0.52M/s](/tpl/images/0525/0164/964d0.png)



