Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 00:30
What is the most stable monatomic ion formed from nitrogen
Answers: 2

Chemistry, 22.06.2019 04:20
Which of the following is true for the actual yield of a reaction? it is always calculated as a ratio. it is the yield from the excess reactant. it is the yield from the limiting reactant. it is always less than the theoretical yield.
Answers: 1

Chemistry, 22.06.2019 07:40
22. a flask containing 450 ml of 0.50 m h2so4 was accidentally knocked to the floor. how many grams of nahco, do you need to put on the spill to neutralize the acid according to the following equation: h2so4(aq)+2 nahcos(aq) na,so(aq) +2 h20()+2 co2(g) d) 38 g a) 2.3 g b) 9.5 g c) 19 g
Answers: 1

Chemistry, 22.06.2019 11:00
Iron (3) oxide will decompose in the presence of hydrogen gas and heater to produced iron and digydrogen monoxide white a balanced chemical equation
Answers: 1
You know the right answer?
The rate constants of some reactions double with every 10 degree rise in temperature. Assume that a...
Questions


Social Studies, 27.02.2021 22:00

History, 27.02.2021 22:00


Mathematics, 27.02.2021 22:00


Mathematics, 27.02.2021 22:00



Mathematics, 27.02.2021 22:00

Physics, 27.02.2021 22:00

Chemistry, 27.02.2021 22:00

Mathematics, 27.02.2021 22:00


Mathematics, 27.02.2021 22:00

Mathematics, 27.02.2021 22:00


Mathematics, 27.02.2021 22:00


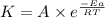
![\log (\frac{K_2}{K_1})=\frac{Ea}{2.303\times R}[\frac{1}{T_1}-\frac{1}{T_2}]](/tpl/images/0524/9978/6d953.png)
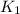 = rate constant at 271 K
= rate constant at 271 K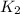 = rate constant at 281 K =
= rate constant at 281 K = 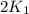
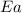 = activation energy for the reaction = ?
= activation energy for the reaction = ?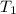 = initial temperature = 271 K
= initial temperature = 271 K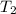 = final temperature = 281 K
= final temperature = 281 K![\log (\frac{2K_1}{K_1})=\frac{Ea}{2.303\times 8.314J/mole.K}[\frac{1}{271K}-\frac{1}{281K}]](/tpl/images/0524/9978/ceafb.png)