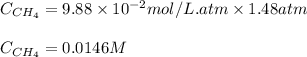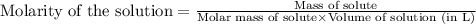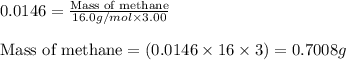Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 17:30
You are performing an experiment in a lab to attempt a new method of producing pure elements from compounds. the only problem is that you do not know what element will form. by your previous calculations you know that you will have 6.3 moles of product. when it is complete, you weigh it and determine you have 604.4 grams. what element have you produced?
Answers: 1


Chemistry, 23.06.2019 10:30
Describe the hybridization of each carbon and nitrogen atom in each of the following structures
Answers: 1

Chemistry, 23.06.2019 16:00
Which is the best metal to use in an alloy to increase its electrical conductivity?
Answers: 2
You know the right answer?
Methane (CH4, molar mass = 16.0 g/mol) has a Henry's Law constant (kH) of 9.88 × 10–2 mol/(L·atm) wh...
Questions

Mathematics, 20.12.2019 03:31

Mathematics, 20.12.2019 03:31

English, 20.12.2019 03:31

Mathematics, 20.12.2019 03:31

Mathematics, 20.12.2019 03:31

Mathematics, 20.12.2019 03:31


Mathematics, 20.12.2019 03:31

Mathematics, 20.12.2019 03:31

Mathematics, 20.12.2019 03:31



History, 20.12.2019 03:31

Mathematics, 20.12.2019 03:31



Biology, 20.12.2019 03:31



Biology, 20.12.2019 03:31

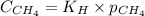
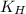 = Henry's constant =
= Henry's constant = 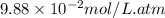
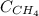 = molar solubility of methane gas = ?
= molar solubility of methane gas = ?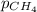 = partial pressure of methane gas = 1.48 atm
= partial pressure of methane gas = 1.48 atm