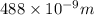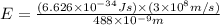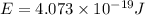Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 03:30
Melting and boiling are endothermic processes. this means that these processes absorb energy from their surroundings in order to occur. use this information and the data you collected in the phase change gizmo to describe what happens to the temperature of water when you boil it, then explain why this result occurs.
Answers: 2

Chemistry, 22.06.2019 05:40
Consider the elements bromine and chlorine; which elements has a larger ionic radius ?
Answers: 1

Chemistry, 22.06.2019 14:10
16. in a reaction that has reached equilibrium, a. the forward and reverse reactions are occurring at the same rate. b. the reactants and products are in equal concentrations. c. the forward reaction has gone further than the reverse reaction. d. there are equal numbers of atoms on both sides of the equation. e. a, b, and d are correct.
Answers: 2

You know the right answer?
What is the energy of a single photon of blue light with a wavelength of 488 nm (488 × 10-9 m)?...
Questions

Arts, 08.04.2021 23:00


Mathematics, 08.04.2021 23:00



Mathematics, 08.04.2021 23:00

Mathematics, 08.04.2021 23:00


Mathematics, 08.04.2021 23:00




Biology, 08.04.2021 23:00

Mathematics, 08.04.2021 23:00

Mathematics, 08.04.2021 23:00

Mathematics, 08.04.2021 23:00


Geography, 08.04.2021 23:00


English, 08.04.2021 23:00

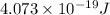
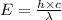
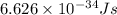
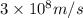
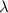 = wavelength =
= wavelength =