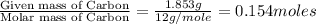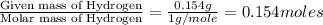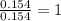Chemistry, 26.02.2020 16:40 kayla942783
A 2.007 g sample of a hydrocarbon is combusted to give 1.389 g of H 2O and 6.785 g of CO 2. What is the empirical formula of the compound? a. CH2 b. C2Hw c. C3H4 d. CH e. C5H12

Answers: 1


Another question on Chemistry



Chemistry, 22.06.2019 21:50
Given the data below for the reaction, 2 a + 2 b + 4 c => d + e + 3 f, the reaction is fill in the [ ] order in a, fill in the [ ] order in b, fill in the [ ] order in c and fill in the [ ] order overall. (use the words "first, second, third, fourth" to fill each blank)experimentinitial conc of a, mol/l initial conc of b, mol/l initial conc of c, mol/l initial rate, mol/l.s1 0.1 0.1 0.2 2 x 10-32 0.2 0.3 0.2 6 x 10-33 0.3 0.1 0.2 2 x 10-34 0.4 0.3 0.4 1.2 x 10-2
Answers: 2

Chemistry, 23.06.2019 04:00
What changes occur in the reaction indicated by the equation? check all that apply. the hydrogen nucleus loses protons. the oxygen nucleus gains protons. the bond in h2 is broken, and new bonds are formed between hydrogen and oxygen atoms. each electron associated with a hydrogen atom is shared with an oxygen atom.
Answers: 3
You know the right answer?
A 2.007 g sample of a hydrocarbon is combusted to give 1.389 g of H 2O and 6.785 g of CO 2. What is...
Questions

Social Studies, 25.04.2021 20:00

Mathematics, 25.04.2021 20:00

Chemistry, 25.04.2021 20:00




Social Studies, 25.04.2021 20:00

Physics, 25.04.2021 20:00


Mathematics, 25.04.2021 20:00


Mathematics, 25.04.2021 20:00


English, 25.04.2021 20:00

Mathematics, 25.04.2021 20:00



Mathematics, 25.04.2021 20:00


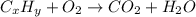
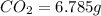
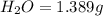
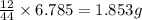 of carbon will be contained.
of carbon will be contained.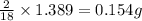 of hydrogen will be contained.
of hydrogen will be contained.