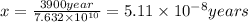Chemistry, 26.02.2020 02:28 ksanquist8896
The activation energy for a reaction is changed from 184 kJ/mol to 59.0 kJ/mol at 600. K by the introduction of a catalyst. If the uncatalyzed reaction takes about 3900 years to occur, about how long will the catalyzed reaction take?

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 03:20
Which type of substance ionizes partially and gives off hydrogen ions when dissolved in water? a. strong acid b. strong base c. weak acid d. weak base
Answers: 1

Chemistry, 22.06.2019 04:20
Which of the following is true for the actual yield of a reaction? it is always calculated as a ratio. it is the yield from the excess reactant. it is the yield from the limiting reactant. it is always less than the theoretical yield.
Answers: 1

Chemistry, 22.06.2019 04:40
*will mark you brainliest + 15 points ** why does the equilibrium of a system shift when the pressure is increased? a. to maximize the stress on the system b. to stop restoring equilibrium to the system c. to increase the total moles of gas in the system d. to decrease the total moles of gas in the system
Answers: 3

Chemistry, 22.06.2019 17:00
The arrangement of particles is most ordered in a sample of
Answers: 1
You know the right answer?
The activation energy for a reaction is changed from 184 kJ/mol to 59.0 kJ/mol at 600. K by the intr...
Questions




Chemistry, 09.01.2020 07:31



Chemistry, 09.01.2020 07:31













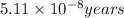 .
.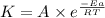
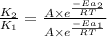
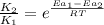
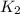 = rate of reaction with catalyst
= rate of reaction with catalyst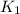 = rate of reaction without catalyst
= rate of reaction without catalyst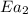 = activation energy with catalyst = 59.0 kJ/mol = 59000 J/mol
= activation energy with catalyst = 59.0 kJ/mol = 59000 J/mol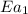 = activation energy without catalyst = 184 kJ/mol = 184000 J/mol
= activation energy without catalyst = 184 kJ/mol = 184000 J/mol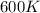
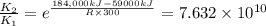
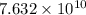 when catalyst is present.
when catalyst is present.