

Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 21:00
Consider the nuclear equation below. 239 > x + 4 he 94 2 what is x? 1.235 cm 96 2.243 u 92 3.235 u 92 4.243 cm 96
Answers: 2

Chemistry, 22.06.2019 10:00
Water's surface tension and heat storage capacity are accounted for by its a) orbitals b) weight c) hydrogen bonds d) mass e) size
Answers: 2

Chemistry, 22.06.2019 12:00
Consider the following reaction at equilibrium. 2co2 (g) 2co (g) + o2 (g) h° = -514 kj le châtelier's principle predicts that the equilibrium partial pressure of co (g) can be maximized by carrying out the reaction a. at high temperature and high pressure b. at high temperature and low pressure c. at low temperature and low pressure d. at low temperature and high pressure e. in the presence of solid carbon
Answers: 2

Chemistry, 22.06.2019 19:00
What information does a complete ionic equation give that the balanced equation doesn’t show?
Answers: 1
You know the right answer?
Ahydrate of cocl2 with a mass of 6.00 g is heated strongly. after cooling the mass the anhydrate is...
Questions

Mathematics, 30.11.2020 22:00




History, 30.11.2020 22:00

Mathematics, 30.11.2020 22:00


Health, 30.11.2020 22:00

Mathematics, 30.11.2020 22:00


Biology, 30.11.2020 22:00


Chemistry, 30.11.2020 22:00



Mathematics, 30.11.2020 22:00

Mathematics, 30.11.2020 22:00



English, 30.11.2020 22:00

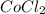 = 6.00 g
= 6.00 g

