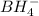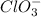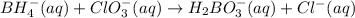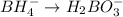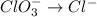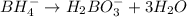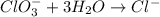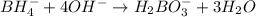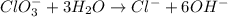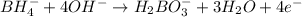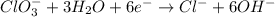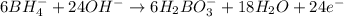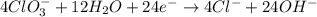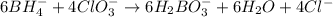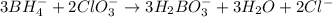Balance the following skeleton reaction and identify the oxidizing and reducing agents: Include the states of all reactants and products in your balanced equation. You do not need to include the states with the identities of the oxidizing and reducing agents.
BH4−(aq) + ClO3−(aq) → H2BO3−(aq) + Cl−(aq) [basic]
a. The oxidizing agent is:
b. The reducing agent is:

Answers: 2


Another question on Chemistry


Chemistry, 22.06.2019 12:30
The bond energy for the van der waals bond between two helium atoms is 7.9×10−4ev. assuming that the average kinetic energy of a helium atom is (3/2)kbt, at what temperature is the average kinetic energy equal to the bond energy between two helium atoms
Answers: 1

Chemistry, 23.06.2019 00:30
What is the percent by mass of magnesium sulfate in mgso4.7h2o
Answers: 3

Chemistry, 23.06.2019 02:00
An alpha particle is: a hydrogen atom a nucleus of helium two neutrons an electron
Answers: 1
You know the right answer?
Balance the following skeleton reaction and identify the oxidizing and reducing agents: Include the...
Questions



Mathematics, 21.10.2020 19:01

Mathematics, 21.10.2020 19:01

History, 21.10.2020 19:01


Mathematics, 21.10.2020 19:01

Health, 21.10.2020 19:01

Mathematics, 21.10.2020 19:01


English, 21.10.2020 19:01

Health, 21.10.2020 19:01



History, 21.10.2020 19:01

Social Studies, 21.10.2020 19:01



Mathematics, 21.10.2020 19:01

Computers and Technology, 21.10.2020 19:01