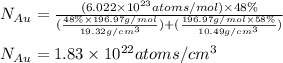Gold forms a substitutional solid solution with silver. Compute the number of gold atoms per cubic centimeter for a silver-gold alloy that contains 42 wt% Au and 58 wt% Ag. The densities of pure gold and silver are 19.32 and 10.49 g/cm3, respectively. The atomic weight of Au is 196.97 g/mol.

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 21:30
In science class richard learns that a substance has a boiling point of 230 fahrenheit his teacher ask him to convert this temperature to degrees celsius what is the boiling point of his substance in degrees celsius
Answers: 3

Chemistry, 22.06.2019 21:30
How many oxygen atoms are there in 3.15 moles of hcl manganese (iv) oxide, mno2
Answers: 2

Chemistry, 22.06.2019 22:00
Choose all the answers that apply. fluorine (f) has an atomic number of 9 and an atomic weight of 18.99. fluorine has a. 9 protons b. 10 neutrons c. 18 electrons d. an atomic mass of 19 e. at least one isotope
Answers: 1

Chemistry, 23.06.2019 04:31
Areaction is first order. if the initial reactant concentration is 0.0200 m, and 25.0 days later the concentration is 6.25 x 10-4 m, then its half-life is:
Answers: 1
You know the right answer?
Gold forms a substitutional solid solution with silver. Compute the number of gold atoms per cubic c...
Questions


History, 28.08.2019 00:00


History, 28.08.2019 00:00



Biology, 28.08.2019 00:00

English, 28.08.2019 00:00



Biology, 28.08.2019 00:00

Mathematics, 28.08.2019 00:00

Mathematics, 28.08.2019 00:00


Mathematics, 28.08.2019 00:00





Social Studies, 28.08.2019 00:00


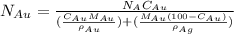
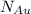 = number of gold atoms per cubic centimeters
= number of gold atoms per cubic centimeters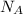 = Avogadro's number =
= Avogadro's number = 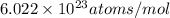
 = Mass percent of gold in the alloy = 42 %
= Mass percent of gold in the alloy = 42 % = Density of pure gold =
= Density of pure gold = 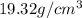
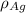 = Density of pure silver =
= Density of pure silver = 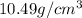
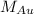 = molar mass of gold = 196.97 g/mol
= molar mass of gold = 196.97 g/mol