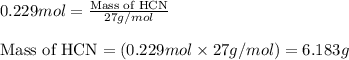Hydrogen cyanide, HCN, is prepared from ammonia, air, and natural gas (CH4) by the following process: Hydrogen cyanide is used to prepare sodium cyanide, which is used in part to obtain gold from gold-containing rock. If a reaction vessel contains 5.90 g NH3, 11.0 g O2, and 4.67 g CH4, what is the maximum mass in grams of hydrogen cyanide that could be made, assuming the reaction goes to completion as written?

Answers: 3


Another question on Chemistry


Chemistry, 22.06.2019 11:30
What is the main reason why some developing countries fear the increase the free trade policies around the world?
Answers: 2

Chemistry, 22.06.2019 21:20
The organs inside the body and how they function together
Answers: 3

Chemistry, 22.06.2019 23:00
What is the oxidation state of an individual bromine atom in nabro3?
Answers: 2
You know the right answer?
Hydrogen cyanide, HCN, is prepared from ammonia, air, and natural gas (CH4) by the following process...
Questions

Mathematics, 08.12.2019 09:31




Health, 08.12.2019 09:31

English, 08.12.2019 09:31

Mathematics, 08.12.2019 09:31

Physics, 08.12.2019 09:31

Mathematics, 08.12.2019 09:31


Mathematics, 08.12.2019 09:31

History, 08.12.2019 09:31


Mathematics, 08.12.2019 09:31

Mathematics, 08.12.2019 09:31

Mathematics, 08.12.2019 09:31

Mathematics, 08.12.2019 09:31

Mathematics, 08.12.2019 09:31


History, 08.12.2019 09:31

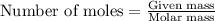 .....(1)
.....(1)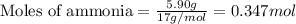
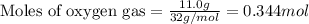
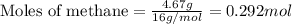
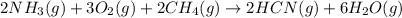
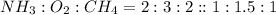
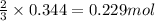 of HCN
of HCN