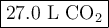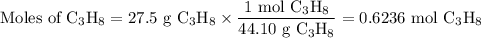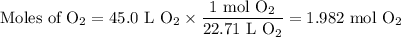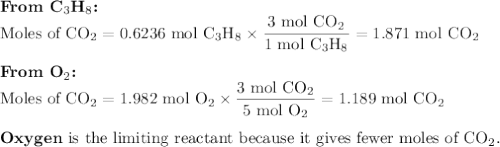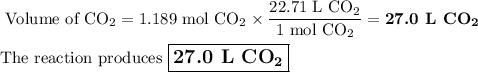The complete combustion of propane (C3H8) in the presence of oxygen yields CO2 and H2O:
C3H8 (...

Chemistry, 25.02.2020 22:19 tiaholmes31
The complete combustion of propane (C3H8) in the presence of oxygen yields CO2 and H2O:
C3H8 (g) + 5O2 (g) 3CO2 (g) + 4H2O (g)
a. Calcluate the volume of carbon dioixde ( at s. t.p.) that would be produced by the
combustion of 27.5 g of C3H8 burns in the presence of 45.0 L of O2.

Answers: 2


Another question on Chemistry


Chemistry, 21.06.2019 22:30
Which of these sequences lists the correct order for the creation of sedimentary rock from sediment? a. deposition, burial, compaction, cementation b. burial, deposition, compaction, cementation c. compaction, deposition, burial, cementation d. cementation, deposition, burial, compaction
Answers: 1

Chemistry, 22.06.2019 09:00
Given the following reaction: c3h8+5o2=3co2+4h20 how many grams of co2 will be produced 7 g of c3h8 and 98 g of o2
Answers: 1

Chemistry, 22.06.2019 12:30
Clyde and marilyn are riding a roller coaster. during which section(s) of the track is their potential energy converted to kinetic energy? a. from point b to point c only b. from point b to point d only c. from point a to point b only d. from point a to point b and from point c to point d
Answers: 1
You know the right answer?
Questions



Chemistry, 02.10.2020 09:01


Biology, 02.10.2020 09:01

Social Studies, 02.10.2020 09:01

English, 02.10.2020 09:01

Mathematics, 02.10.2020 09:01

Mathematics, 02.10.2020 09:01


Mathematics, 02.10.2020 09:01

Biology, 02.10.2020 09:01


Mathematics, 02.10.2020 09:01

Mathematics, 02.10.2020 09:01


English, 02.10.2020 09:01


Physics, 02.10.2020 09:01