
Chemistry, 25.02.2020 21:45 Kelshonti15
For the reaction, A(g) + B(g) => AB(g), the rate is 0.625 mol/L. s when the initial concentrations of both A and B are 2.00 mol/L. If the reaction is first order in A and first order in B, what is the rate when the initial concentration of A = 4.41 mol/L and that of B = 2.72 mol/L. Give answer to 2 decimal places.

Answers: 1


Another question on Chemistry


Chemistry, 22.06.2019 19:30
Which one of the following substances would be the most soluble in ccl4? na2so4 h2o ch3ch2ch2ch2oh c4h10 hi
Answers: 1

Chemistry, 22.06.2019 20:30
The activation energy for the reaction no2(g)+co2(g)⟶no(g)+co(g) is ea = 300 kj/mol and the change in enthalpy for the reaction is δh = -100 kj/mol . what is the activation energy for the reverse reaction?
Answers: 3

Chemistry, 23.06.2019 03:30
Astudent uses universal ph paper to find the ph of three solutions . solution a has a ph of 5 solution b has a ph of 11 and solution c has a ph of 7 identify which solution is acidic which solution is neutral and which solution is basic
Answers: 1
You know the right answer?
For the reaction, A(g) + B(g) => AB(g), the rate is 0.625 mol/L. s when the initial concentration...
Questions


Biology, 26.01.2022 04:20




Mathematics, 26.01.2022 04:20




Mathematics, 26.01.2022 04:20

Chemistry, 26.01.2022 04:20


Biology, 26.01.2022 04:20







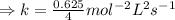
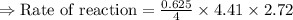
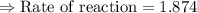 ≈1.87 mol L⁻¹s⁻¹
≈1.87 mol L⁻¹s⁻¹



