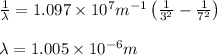Consider a transition of the electron in the hydrogen atom from n=3 to n=7.
a) Determine...


Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 02:30
Margaret wants to make an orange flavored drink by stirring powdered drink mix into a glass of water. she doesn't like drinks that have small clumps of powdered solid in them, so she wants the drink to be a perfect solution. what factors should margaret not consider when deciding how much powder to add to her glass of water?
Answers: 3

Chemistry, 22.06.2019 15:00
Describe what happens to the molecules as water goes from ice to liquid to vapor. be sure to explain what happens to the temperature during the phase changes.
Answers: 2

Chemistry, 22.06.2019 16:30
Explain in detail of the four major scientific developments that spurred the formulation of the plate tectonics theory
Answers: 2

Chemistry, 22.06.2019 19:00
How many moles of cu are needed to react with 5.8 moles of agno3? cu + 2 agno3 → cu(no3)2 + 2 ag
Answers: 3
You know the right answer?
Questions

English, 21.08.2019 13:50

Mathematics, 21.08.2019 13:50







English, 21.08.2019 13:50

Mathematics, 21.08.2019 13:50


Chemistry, 21.08.2019 13:50





Social Studies, 21.08.2019 13:50

Mathematics, 21.08.2019 13:50

History, 21.08.2019 13:50

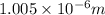
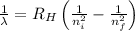
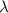 = Wavelength of radiation
= Wavelength of radiation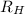 = Rydberg's Constant =
= Rydberg's Constant = 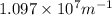
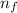 = Higher energy level = 7
= Higher energy level = 7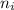 = Lower energy level = 3
= Lower energy level = 3