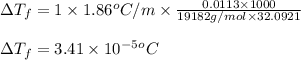A biochemical engineer isolates a bacterial gene fragment and dissolves an 11.3 mg sample of the material in enough water to make 32.2 mL of solution. The osmotic pressure of the solution is 0.340 torr at 25°C.
(a) What is the molar mass of the gene fragment?
(b) If the solution density is 0.997 g/mL, how large is the freezing point depression for this solution (Kf of water = 1.86 °C/m)?

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 02:00
What is the maximum number of electrons that an atomic orbital can contain?
Answers: 1


Chemistry, 22.06.2019 21:20
The organs inside the body and how they function together
Answers: 3

Chemistry, 22.06.2019 21:20
40dm3 of gas at 760 torr are heated from 5°c to 50°c what is the new volume
Answers: 3
You know the right answer?
A biochemical engineer isolates a bacterial gene fragment and dissolves an 11.3 mg sample of the mat...
Questions

Chemistry, 13.04.2021 22:30




Mathematics, 13.04.2021 22:30

Mathematics, 13.04.2021 22:30



Mathematics, 13.04.2021 22:30


Spanish, 13.04.2021 22:30




Biology, 13.04.2021 22:30

Mathematics, 13.04.2021 22:30


Mathematics, 13.04.2021 22:30


Mathematics, 13.04.2021 22:30

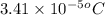
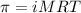
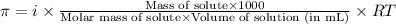
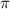 = osmotic pressure of the solution = 0.340 torr
= osmotic pressure of the solution = 0.340 torr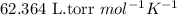
![25^oC=[273+25]=298K](/tpl/images/0523/6100/6a9f9.png)

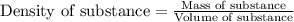
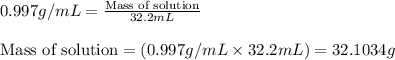
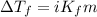
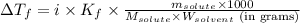
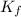 = molal freezing point elevation constant = 1.86°C/m
= molal freezing point elevation constant = 1.86°C/m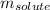 = Given mass of solute (gene fragment) = 0.0113 g
= Given mass of solute (gene fragment) = 0.0113 g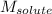 = Molar mass of solute (gene fragment) = 19182 g/mol
= Molar mass of solute (gene fragment) = 19182 g/mol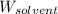 = Mass of solvent (water) = 32.0921 g
= Mass of solvent (water) = 32.0921 g