Chemistry, 25.02.2020 19:47 kaylailkanic1487
Be sure to answer all parts. For the titration of 10.0 mL of 0.250 M acetic acid with 0.200 M sodium hydroxide, determine the pH when: (a) 10.0 mL of base has been added. (b) 12.5 mL of base has been added. (c) 15.0 mL of base has been added.

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 01:00
Agas occupies 475 cm^3 at 313k. find its volume at 367k. you must show all of your work to receive credit. be sure to identify which of the gas laws you will be using
Answers: 2

Chemistry, 22.06.2019 12:30
Which statement is true about this reaction? 14n+1h 15o it is a practical source of energy on earth. it occurs only outside the solar system. its product is heavier than each of its reactants. it shows the critical mass of an element.
Answers: 2

Chemistry, 22.06.2019 19:30
Helium decays to form lithium. which equation correctly describes this decay?
Answers: 2

Chemistry, 23.06.2019 08:30
Explain how to convert from one unit to another in the metric system.
Answers: 3
You know the right answer?
Be sure to answer all parts. For the titration of 10.0 mL of 0.250 M acetic acid with 0.200 M sodium...
Questions

Spanish, 25.10.2019 01:43

History, 25.10.2019 01:43








Mathematics, 25.10.2019 01:43








Mathematics, 25.10.2019 01:43


Mathematics, 25.10.2019 01:43

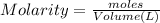
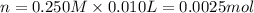
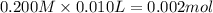
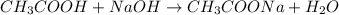
![[H^+]=\frac{0.0005 mol}{0.020 L}=0.025 M](/tpl/images/0523/5054/d4db0.png)
![pH=-\log[H^+]=-\log[0.025 M]=1.60](/tpl/images/0523/5054/f1f2d.png)
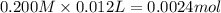
![[H^+]=\frac{0.0001 mol}{0.022 L}=0.0045 M](/tpl/images/0523/5054/5492d.png)
![pH=-\log[H^+]=-\log[0.0045 M]=2.34](/tpl/images/0523/5054/d5f92.png)
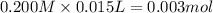
![[OH^-]=\frac{0.0005 mol}{0.025 L}=0.02 M](/tpl/images/0523/5054/d1bd6.png)
![pOH=-\log[OH^-]=-\log[0.02 M]=1.70](/tpl/images/0523/5054/8bd06.png)