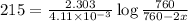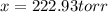Dinitrogen pentoxide decomposes in the gas phase to form nitrogen dioxide and oxygen gas. The reaction is first order in dinitrogen pentoxide and has a half-life of 2.81 h at 25 ∘C. If a 1.7 −L reaction vessel initially contains 755 torr of N2O5 at 25 ∘C, what partial pressure of O2 will be present in the vessel after 215 minutes?

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 12:00
Which of the following is an example of physical change not a chemical change? a) a log gives off heat and light as it burns. b) a tree stores energy from the sun in its fruit. c) a penny lost in the grass slowly changes color. d) a water pipe freezes and cracks on a cold night.
Answers: 2

Chemistry, 23.06.2019 00:00
The empirical formula of a compound is ch2o and its mass is 120 amu/molecule, what is its formula?
Answers: 1

Chemistry, 23.06.2019 01:00
Which is true concerning the products and reactants of photosynthesis and cellular respiration? a. the products of photosynthesis are sugars and the reactants of cellular respiration are starches. b. the products of photosynthesis are reactants in cellular respiration. c. oxygen is needed for photosynthesis and is given off in cellular respiration.
Answers: 2

Chemistry, 23.06.2019 04:20
Calculate the mass of 0.750 mol of the following substance. na3po4.
Answers: 1
You know the right answer?
Dinitrogen pentoxide decomposes in the gas phase to form nitrogen dioxide and oxygen gas. The reacti...
Questions

Biology, 05.10.2019 11:30

Engineering, 05.10.2019 11:30

Health, 05.10.2019 11:30






Computers and Technology, 05.10.2019 11:30




Computers and Technology, 05.10.2019 11:30


Computers and Technology, 05.10.2019 11:30


Computers and Technology, 05.10.2019 11:30

Computers and Technology, 05.10.2019 11:30

Computers and Technology, 05.10.2019 11:30

Computers and Technology, 05.10.2019 11:30

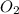 is, 222.93 torr
is, 222.93 torr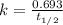
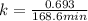
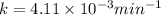
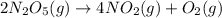
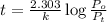
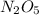 = 760 torr
= 760 torr