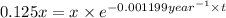Answers: 3


Another question on Chemistry


Chemistry, 22.06.2019 06:40
Which statement correctly describes metallic bonds? a. they form when certain atoms lose electrons and other atoms gain electrons. b. they involve an attraction between anions and cations. they always involvpoth a metal and a nonmetal. d. they can only form between atoms of the same element. e. they form because electrons can move freely between atoms.
Answers: 3

Chemistry, 22.06.2019 11:00
Which statement correctly identifies the scientific question and describes why the question is scientific? question 1 refers to the supernatural.question 2 reflects a moral or social value.question 3 refers to something that can be measured.question 4 reflects a question that can’t be observed.
Answers: 1

Chemistry, 22.06.2019 14:10
16. in a reaction that has reached equilibrium, a. the forward and reverse reactions are occurring at the same rate. b. the reactants and products are in equal concentrations. c. the forward reaction has gone further than the reverse reaction. d. there are equal numbers of atoms on both sides of the equation. e. a, b, and d are correct.
Answers: 2
You know the right answer?
A decomposition reaction has a half life of 578 years. (a) What is the rate constant for this reacti...
Questions



Mathematics, 19.05.2020 13:11

Mathematics, 19.05.2020 13:11





Mathematics, 19.05.2020 13:11

Mathematics, 19.05.2020 13:11

Mathematics, 19.05.2020 13:11

Mathematics, 19.05.2020 13:11





Social Studies, 19.05.2020 13:11

English, 19.05.2020 13:11

Mathematics, 19.05.2020 13:11

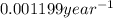 is the rate constant for this reaction.
is the rate constant for this reaction.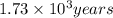 to concentration to reach 12.5% of its original value.
to concentration to reach 12.5% of its original value.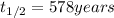
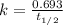
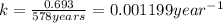
![[A_o]](/tpl/images/0522/9110/dc622.png) = x
= x![[A]](/tpl/images/0522/9110/6aa06.png) = 12.5% of x = 0.125x
= 12.5% of x = 0.125x![[A]=[A_o]\times e^{-kt}](/tpl/images/0522/9110/abdec.png)