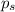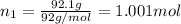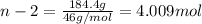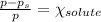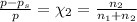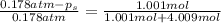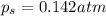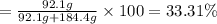Chemistry, 25.02.2020 05:17 paytonxxburns05
An ideal solution containing 92.1 g of glycerin, C3H5(OH)3, and 184.4 g of ethanol, C2H5OH, is at 40°C. The vapor pressure of pure ethanol is 0.178 atm at 40°C. Glycerin is essentially nonvolatile at this temperature. Compute the vapor pressure and weight percentage of Glycerin.

Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 21:30
It takes 945.kj/mol to break a nitrogen-nitrogen triple bond. calculate the maximum wavelength of light for which a nitrogen-nitrogen triple bond could be broken by absorbing a single photon.
Answers: 3

Chemistry, 21.06.2019 23:00
The drawing represents the movement of particles in a substance. what changes of state can this substance undergo
Answers: 1


Chemistry, 22.06.2019 12:00
What term is applied to a scientist who studies ancient life, including animal and plant fossils a. anthropologist b. dendroclimatologist c. geophysicist d. paleontologist
Answers: 2
You know the right answer?
An ideal solution containing 92.1 g of glycerin, C3H5(OH)3, and 184.4 g of ethanol, C2H5OH, is at 40...
Questions



English, 31.10.2020 04:50


Mathematics, 31.10.2020 04:50

Arts, 31.10.2020 04:50

Social Studies, 31.10.2020 04:50

Mathematics, 31.10.2020 04:50

Mathematics, 31.10.2020 04:50




Social Studies, 31.10.2020 04:50

Mathematics, 31.10.2020 04:50

Arts, 31.10.2020 04:50


Business, 31.10.2020 04:50

Mathematics, 31.10.2020 04:50

Mathematics, 31.10.2020 04:50

Mathematics, 31.10.2020 04:50