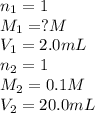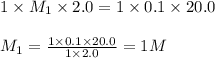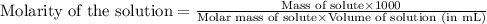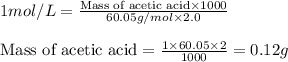Chemistry, 25.02.2020 05:12 rosetoheart2
You have a 2.0 mL sample of acetic acid (molar mass 60.05 g/mol) of unknown concentration. You titrate it to its endpoint with 20.0 mL NaOH (0.1 M). What mass of acetic acid was dissolved in the 2.0 mL of solution used?

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 22:00
Which produce would best increase the amount of heat energy that is actually gained by calorimeter b
Answers: 1

Chemistry, 22.06.2019 13:30
Ants live on acacia trees in south america. the ants feed on sugars secreted by the trees. the trees provide room for the ants to live. the ants sting any other insect or animal that comes to eat the trees. what type of relationship is this?
Answers: 1

Chemistry, 22.06.2019 18:00
An object displaces 652 ml of water. the volume of the object is: 0.652 cm³ 6.52 cm³ 65.2 cm³ 652 cm³
Answers: 2

Chemistry, 22.06.2019 18:30
The number of moles of a given mass of a substance can be found without knowing its molecular formula or molar mass. true false
Answers: 1
You know the right answer?
You have a 2.0 mL sample of acetic acid (molar mass 60.05 g/mol) of unknown concentration. You titra...
Questions

English, 17.10.2021 18:40




English, 17.10.2021 18:40



Mathematics, 17.10.2021 18:40

History, 17.10.2021 18:40


English, 17.10.2021 18:40



Computers and Technology, 17.10.2021 18:40



Mathematics, 17.10.2021 18:40

Mathematics, 17.10.2021 18:40

Mathematics, 17.10.2021 18:40

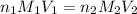
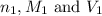 are the n-factor, molarity and volume of acid which is
are the n-factor, molarity and volume of acid which is 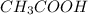
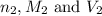 are the n-factor, molarity and volume of base which is NaOH.
are the n-factor, molarity and volume of base which is NaOH.