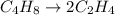Chemistry, 25.02.2020 03:51 locomexicano03
The reaction C4H8 → 2 C2H4 has a rate constant of 2.25×10–2 s –1 . What is [C2H4 ] after 15.0 s if the reaction container initially contained only C4H8 at an initial concentration of 0.500 M?

Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 18:00
The compound methyl butanoate smells like apples. its percent composition is 58.8% c, 9.9% h, and 31.4% o. what’s the empirical formula ?
Answers: 1


Chemistry, 22.06.2019 22:30
Vi limitens. vastery test select the correct answer. which statement explains why large atoms are more reactive than small atoms? a. large atoms have valence electrons farther from the nucleus and lose them more readily. b. large atoms have greater ionization energy, which they can utilize during a reaction. c. large atoms have a greater number of electrons that they can lose during a reaction. d. large atoms have more energy levels, so they have more energy to pass on in a reaction. reset next
Answers: 3

Chemistry, 23.06.2019 00:30
Gasoline has a density of 0.740 g/ml. if you have 328 grams of gasoline, what is the volume in milliliters?
Answers: 1
You know the right answer?
The reaction C4H8 → 2 C2H4 has a rate constant of 2.25×10–2 s –1 . What is [C2H4 ] after 15.0 s if t...
Questions

Mathematics, 25.05.2021 22:20

Social Studies, 25.05.2021 22:20

Social Studies, 25.05.2021 22:20



English, 25.05.2021 22:20

Mathematics, 25.05.2021 22:20

Mathematics, 25.05.2021 22:20

Mathematics, 25.05.2021 22:20


Mathematics, 25.05.2021 22:20

Physics, 25.05.2021 22:20

English, 25.05.2021 22:20

Social Studies, 25.05.2021 22:20


English, 25.05.2021 22:20

History, 25.05.2021 22:20

Mathematics, 25.05.2021 22:20


Biology, 25.05.2021 22:20

![k=\frac{2.303}{t}\log\frac{[A_o]}{[A]}](/tpl/images/0522/7034/f1041.png)
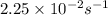
![[A_o]](/tpl/images/0522/7034/dc622.png) = initial amount of the reactant = 0.500 M
= initial amount of the reactant = 0.500 M![2.25\times 10^{-2}s^{-1}=\frac{2.303}{15.0s}\log\frac{0.500}{[A]}](/tpl/images/0522/7034/20174.png)
![[A]=0.357M](/tpl/images/0522/7034/b55f6.png)