
Chemistry, 25.02.2020 03:23 tdyson3p6xvtu
Calculate the standard heat of reaction for the following methane-generating reaction of methanogenic bacteria: 4CH3NH2(g) + 2H2O(l) → 3CH4(g) + CO2(g) + 4NH3(g) Given that ΔHfo(CH3NH2, g) = –22.97 kJ/mol; ΔHfo(H2O, l) = –285.8 kJ/mol; ΔHfo(CH4, g) = –74.8 kJ/mol; ΔHfo(CO2, g) = –393.5 kJ/mol ΔHfo(NH3, g) = –46.1 kJ/mol

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 01:40
C3h8o3 - glycerol major species present when dissolved in water
Answers: 2

Chemistry, 22.06.2019 09:00
This chart lists four kinds of polymers and their sources. what can be known about all four polymers, despite their differences? they come from living things. they share ionic carbon bonds. they are at least 100 monomers long. they are made of repeating subunits.
Answers: 1

Chemistry, 22.06.2019 09:40
Sulfur dioxide and oxygen react to form sulfur trioxide during one of the key steps in sulfuric acid synthesis. an industrial chemist studying this reaction fills a 25.0l tank with 4.5 mol of sulfur dioxide gas and 4.5 mol of oxygen gas at 30.°c. he then raises the temperature, and when the mixture has come to equilibrium measures the amount of sulfur trioxide gas to be 1.4 mol. calculate the concentration equilibrium constant for the reaction of sulfur dioxide and oxygen at the final temperature of the mixture. round your answer to 2 significant digits.
Answers: 3

Chemistry, 22.06.2019 14:00
The two naturally occurring isotopes of chlorine are 35cl (34.969 amu, 75.77%) and 37cl (36.966 amu, 24.23%). the two naturally occurring isotopes of bromine are 79br (78.918 rm amu, 50.69%) and 81br (80.916 amu, 49.31%). chlorine and bromine combine to form bromine monochloride, brcl. 1. how many peaks will be present in a mass spectrum for brcl? the four combinations of molecule possible given these four isotopes are: 81br37cl, 81br35cl, 79br37cl, and 79br35cl. 2. what are the masses of the four different brcl molecules? express the masses using six significant figures, in decreasing numeric order (highest to lowest), separated by commas.
Answers: 3
You know the right answer?
Calculate the standard heat of reaction for the following methane-generating reaction of methanogeni...
Questions

French, 23.04.2021 07:50

Mathematics, 23.04.2021 07:50

Mathematics, 23.04.2021 07:50

Mathematics, 23.04.2021 07:50

English, 23.04.2021 07:50


Spanish, 23.04.2021 07:50


History, 23.04.2021 07:50





Mathematics, 23.04.2021 07:50


Mathematics, 23.04.2021 07:50


Mathematics, 23.04.2021 07:50

Mathematics, 23.04.2021 07:50

Mathematics, 23.04.2021 07:50

![\Delta H^o_{rxn}=\sum [n\times \Delta H_f_{(product)}]-\sum [n\times \Delta H_f_{(reactant)}]](/tpl/images/0522/6322/e893d.png)
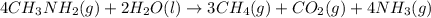
![\Delta H_{rxn}=[(3\times \Delta H_f_{(CH_4(g))})+(1\times \Delta H_f_{(CO_2(g))})+(4\times \Delta H_f_{(NH_3(g))})]-[(4\times \Delta H_f_{(CH_3NH_2(g))})+(2\times \Delta H_f_{(H_2O(l))})]](/tpl/images/0522/6322/6730f.png)
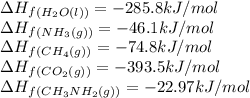
![\Delta H_{rxn}=[(3\times (-74.8))+(1\times (-393.5))+(4\times (-46.1))]-[(4\times (-22.97))+(2\times (-285.8))]\\\\\Delta H_{rxn}=-138.82kJ](/tpl/images/0522/6322/fdc01.png)


