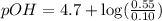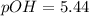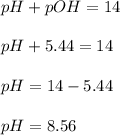Chemistry, 25.02.2020 02:57 fangirl2837
What is the pH of a solution that results when 0.010mol HNO3 is added to 500.ml of a solution that is 0.10M in aqueous ammonia and 0.55 M in ammonium nitrate. assume no volume change. (The Kb for NH3 =1.8 * 10-5 )

Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 20:30
10. translate each of the following chemical equations into a sentence. a. 2 zns(s) + 3 o2(g) -> 2 zno(s) + 2 so2(g) b. cah2(s) + 2 h2o(l) -> ca(oh)2 (aq) + 2 h2(g)
Answers: 2

Chemistry, 22.06.2019 01:00
Water is important for the of cells. a: size, shape, and temperature b: temperature, color, and odor c: color, odor, and size d: shape, temperature, and color
Answers: 2

Chemistry, 22.06.2019 02:30
Select each correct answer. more than one answer may be correct. which of the following is a characteristic of unicellular organisms? they can possess tissues and organs. all of their functions are performed by a single cell. they are usually microscopic. each of their cells is specialized to perform a specific function.
Answers: 1

Chemistry, 22.06.2019 05:30
What is the mass defect of a mole of nuclei with 1.8 x 10^15 j/mol binding energy?
Answers: 1
You know the right answer?
What is the pH of a solution that results when 0.010mol HNO3 is added to 500.ml of a solution that i...
Questions


Arts, 18.06.2020 19:57

English, 18.06.2020 19:57


Mathematics, 18.06.2020 19:57

Social Studies, 18.06.2020 19:57




English, 18.06.2020 19:57




Mathematics, 18.06.2020 19:57


Mathematics, 18.06.2020 19:57

Social Studies, 18.06.2020 19:57



Mathematics, 18.06.2020 19:57

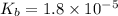
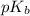 .
.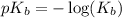
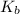 in this expression, we get:
in this expression, we get: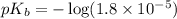
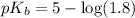
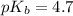
![pOH=pK_b+\log \frac{[Salt]}{[Base]}](/tpl/images/0522/5805/ac570.png)