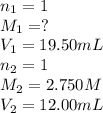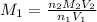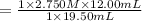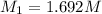Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 09:30
Apump contains 0.5 l of air at 203 kpa.you draw back on the piston of the pump, expanding the volume until the pressure reads 50.8 kpa. what is the new volume of the air pump
Answers: 2

Chemistry, 22.06.2019 17:00
Astable electron arrangement for an atom is one that does not easily change. how is this arrangement arrived at? a. valence electrons are transferred or shared to create a full outer shell of electrons. b. valence electrons are discarded into space to create a full outer shell of electrons. c. protons (positive charge) pair with valence electrons (negative charge) to create a strong bond. d. outer shells with valence electrons are transferred or shared.
Answers: 2

Chemistry, 22.06.2019 23:00
What element has similar physical and chemical properties as boron.
Answers: 1

Chemistry, 23.06.2019 07:00
The image compares the arrangement of electrons in two different neutral atoms. a figure labeled atom q has a shaded sphere at the center of three concentric circles. the innermost circle has two black spheres. the middle circle has six black spheres. to the left of this figure is another figure labeled atom p. atom p has a shaded sphere at the center of three concentric circles. the innermost circle has two black spheres. the middle circle has seven black spheres. which of the following best explains the position of the two atoms in the periodic table?
Answers: 2
You know the right answer?
A student receives a solution of 2.750 M NaOH to titrate with 12.00 mL of an unknown solution of HCl...
Questions


Biology, 29.12.2020 02:30

Mathematics, 29.12.2020 02:30

Mathematics, 29.12.2020 02:30

Mathematics, 29.12.2020 02:30



Geography, 29.12.2020 02:30


English, 29.12.2020 02:30


Mathematics, 29.12.2020 02:30




Mathematics, 29.12.2020 02:40


Biology, 29.12.2020 02:40

Computers and Technology, 29.12.2020 02:40


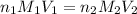
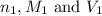 are the n-factor, molarity and volume of acid which is
are the n-factor, molarity and volume of acid which is 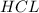
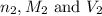 are the n-factor, molarity and volume of base which is NaOH.
are the n-factor, molarity and volume of base which is NaOH.