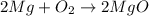Chemistry, 25.02.2020 03:04 tonimgreen17p6vqjq
A student burned 0.250 g of magnesium metal in an open crucible. Oxygen from the air combined with the metal forming magnesium oxide. If 0.421 g of magnesium oxide were produced, what mass of oxygen combined with the metal? 1. 0.171 g 2. 0.671 g 3. 1.684 g 4. 0.594 g

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 17:40
Which statement about hf is true? it is zero for any compound in its standard state. it is positive when the bonds of the product store more energy than those of the reactants. it is negative when a compound forms from elements in their standard states. it is zero for any element that is in the liquid state.
Answers: 1


Chemistry, 23.06.2019 02:30
Apound is approximately 0.45 kilogram. a persons weighs 87 kilograms. what is the persons’s weight, in pounds, when expressed to the correct number of significant figures
Answers: 1

Chemistry, 23.06.2019 04:00
Calculate the mass of 0.750 mol of the following substance. na3po4. , i'm not quite sure on how to set up the problem to solve! : (
Answers: 1
You know the right answer?
A student burned 0.250 g of magnesium metal in an open crucible. Oxygen from the air combined with t...
Questions

Mathematics, 19.09.2019 16:00


Mathematics, 19.09.2019 16:00


Social Studies, 19.09.2019 16:00

Geography, 19.09.2019 16:00

Mathematics, 19.09.2019 16:00


History, 19.09.2019 16:00


Mathematics, 19.09.2019 16:00


Mathematics, 19.09.2019 16:00

Physics, 19.09.2019 16:00


Geography, 19.09.2019 16:00



Mathematics, 19.09.2019 16:00

History, 19.09.2019 16:00