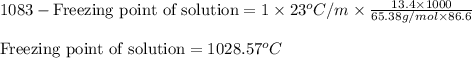Chemistry, 24.02.2020 23:59 loudenalexisp56lp0
The molal freezing point constant for copper is 23 °C/m. If pure copper melts at 1083°C, what will be the melting point of a brass made of 13.4 mass percent Zn and the remainder Cu?

Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 18:00
How is the composition of a meteorite relevant to finding out the composition of earth's core?
Answers: 3

Chemistry, 22.06.2019 05:00
Choose all the answers that apply. ionic compounds dissolve easily in water do not dissolve in water have low melting points have high melting points conduct electricity when melted
Answers: 1

Chemistry, 22.06.2019 06:00
How many atoms of mg are present in 97.22 grams of mg? 6.022 × 1023 2.408 × 1024 4.818 × 1024 5.855 × 1025
Answers: 3

Chemistry, 22.06.2019 08:40
For each of the following compounds, write the formula then predict whether it would be a strong, weak, or non-electrolyte when placed in di water. for the ionic compounds only, put (s) or (aq) after the forrmula formula strong, weak or non electrolyte? a calcium hydroxide b. silver carbonate c. lead(ii) sulfate d. phosphorus trifluoride e. sodium phosphide f barium sulfate g. strontium acetate h. zinc nitrate
Answers: 3
You know the right answer?
The molal freezing point constant for copper is 23 °C/m. If pure copper melts at 1083°C, what will b...
Questions


Mathematics, 20.04.2021 21:10


Mathematics, 20.04.2021 21:10







Health, 20.04.2021 21:10

Mathematics, 20.04.2021 21:10




English, 20.04.2021 21:10


Mathematics, 20.04.2021 21:10


Mathematics, 20.04.2021 21:10

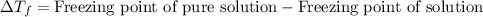
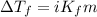
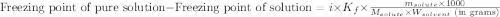
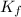 = molal freezing point elevation constant = 23°C/m
= molal freezing point elevation constant = 23°C/m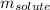 = Given mass of solute (zinc) = 13.4 g
= Given mass of solute (zinc) = 13.4 g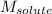 = Molar mass of solute (zinc) = 65.38 g/mol
= Molar mass of solute (zinc) = 65.38 g/mol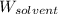 = Mass of solvent (copper) = 86.6 g
= Mass of solvent (copper) = 86.6 g