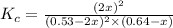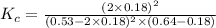Consider the reaction between NONO and Cl2Cl2 to form NOClNOCl: 2NO(g)+Cl2(g)⇌2NOCl(g)2NO(g)+Cl2(g) ⇌2NOCl(g) A reaction mixture at a certain temperature initially contains only [NO]=[NO]= 0.53 MM and [Cl2]=[Cl2]= 0.64 MM. After the reaction comes to equilibrium, the concentration of NOClNOCl is 0.36 MM. Part A Find the value of the equilibrium constant (Kc)(Kc) at this temperature.

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 22:30
Draw the aromatic compound toluene (methylbenzene). show all hydrogen atoms, including those on the ring.
Answers: 1

Chemistry, 22.06.2019 22:30
Which of these statements best explains why space exploration should be encouraged? it prepares humans to live without oxygen. it dispel myths about objects in space. it prevents comets and asteroids from striking earth. it creates technology to absorb harmful radiations in space.
Answers: 1

Chemistry, 23.06.2019 01:00
An unsaturated hydrocarbon is a hydrogen-carbon compound with a. a network solid structure b. single bonds c. single bonds in a branched-chain structure d. double or triple bonds
Answers: 1

Chemistry, 23.06.2019 07:40
Which of the following has expanded our knowledge of the universe beyond our solar system the most? a. manned space travel b. the hubble space telescope c. the pioneer and voyager missions d. the international space station
Answers: 3
You know the right answer?
Consider the reaction between NONO and Cl2Cl2 to form NOClNOCl: 2NO(g)+Cl2(g)⇌2NOCl(g)2NO(g)+Cl2(g)...
Questions


Chemistry, 16.01.2022 14:50

Social Studies, 16.01.2022 14:50


Mathematics, 16.01.2022 14:50

History, 16.01.2022 14:50


Mathematics, 16.01.2022 15:00

Engineering, 16.01.2022 15:00



Mathematics, 16.01.2022 15:00


History, 16.01.2022 15:00

English, 16.01.2022 15:00




World Languages, 16.01.2022 15:00

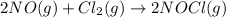
![K_c=\frac{[NOCl]^2}{[NO]^2[Cl_2]}](/tpl/images/0521/6978/56950.png)