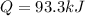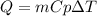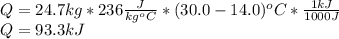Chemistry, 24.02.2020 20:42 TrueKing184
What is the heat needed to raise the temperature of 24.7 kg silver from 14.0 degrees Celsius to 30.0 degrees Celsius? Specific heat capacity of silver is 236 J/(kg * C°)

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 22:30
1. combine iron and copper (ii) sulfate solution. (hint: iron will form the iron (iii) ion) fe + cuso4 → 2. combine lead (ii) nitrate and potassium iodide solutions. pb(no3)2+ kl → 3. combine magnesium metal and hydrochloric acid solution. mg + hcl → 4. electrolysis (splitting) of water. h2o → 5. burning magnesium. mg + o2 →
Answers: 3


Chemistry, 22.06.2019 14:30
Need ! asap will mark 10 pts using the room temperature line (orange line) and your periodic table, make lists that identify the state of matter (gas, liquid, or solid) in which each element you plotted exists at room temperature. explain your answers
Answers: 1

Chemistry, 22.06.2019 19:00
How does kepler second law of planetary motion overthrow one of the basic beliefs of classical astronomy
Answers: 1
You know the right answer?
What is the heat needed to raise the temperature of 24.7 kg silver from 14.0 degrees Celsius to 30.0...
Questions

English, 12.04.2021 04:30

French, 12.04.2021 04:30

Mathematics, 12.04.2021 04:30

Mathematics, 12.04.2021 04:30

Mathematics, 12.04.2021 04:30

History, 12.04.2021 04:30


World Languages, 12.04.2021 04:30

Mathematics, 12.04.2021 04:30

Advanced Placement (AP), 12.04.2021 04:30

French, 12.04.2021 04:30



Mathematics, 12.04.2021 04:30


Mathematics, 12.04.2021 04:30

Mathematics, 12.04.2021 04:30


History, 12.04.2021 04:30

Mathematics, 12.04.2021 04:30