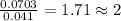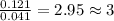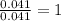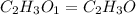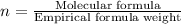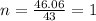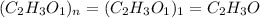Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 19:30
The ph of carrots are 5.0 how it is classified a.acidic b.basic c.indicator d.neutral
Answers: 2

Chemistry, 21.06.2019 20:30
If a bottle of olive oil contains 1.4 kg of olive oil, what is the volume, in milliliters ( ml ), of the olive oil?
Answers: 1

Chemistry, 22.06.2019 00:40
During which time interval does the object travel approximately 10 meters
Answers: 3

Chemistry, 22.06.2019 10:50
Determine the empirical formula for succinic acid that is composed of 40.60% carbon, 5.18% hydrogen, and 54.22% oxygen.
Answers: 1
You know the right answer?
A compound is composed of C, H and O. A 1.621 g sample of this compound was combusted, producing 1.9...
Questions

English, 05.07.2019 05:00



Health, 05.07.2019 05:00

Health, 05.07.2019 05:00

Health, 05.07.2019 05:00

Health, 05.07.2019 05:00


Social Studies, 05.07.2019 05:00

Social Studies, 05.07.2019 05:00

Health, 05.07.2019 05:00

Health, 05.07.2019 05:00

Health, 05.07.2019 05:00

Health, 05.07.2019 05:00


Health, 05.07.2019 05:00


Health, 05.07.2019 05:00

Biology, 05.07.2019 05:00

Biology, 05.07.2019 05:00

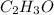
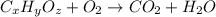
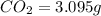
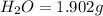
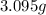 of carbon dioxide,
of carbon dioxide, 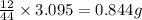 of carbon will be contained.
of carbon will be contained.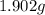 of water,
of water, 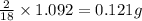 of hydrogen will be contained.
of hydrogen will be contained.![(1.621)-[(0.844)+(0.121)]=0.656g](/tpl/images/0521/3218/1f9d2.png)
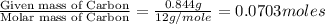
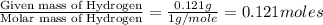
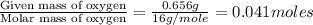
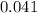 moles.
moles.