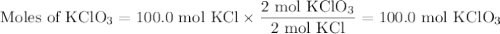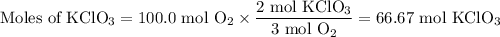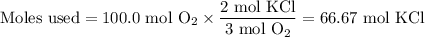Chemistry, 24.02.2020 07:21 DASASDAEDWEDA
Consider the following chemical reaction: 2KCl + 3O2 --> 2KClO3. If you are given 100.0 moles of KCl and 100.0 moles of O2...
what is the limiting reactant? (TYPE EITHER KCl or O2)
what is the excess reactant? (TYPE EITHER KCl or O2)
how many moles of the excess reactant will be left over? moles (TYPE just the number to the correct amount of significant figures)

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 07:30
Plz mark brainliest 30 points 1) find the momentum of a 12 kg snowball that is rolling with a velocity of 9 m/s. 2) an 8 ball with a mass of .5 kg is sitting at rest. it is hit by the cue ball (1 kg) traveling at 2.5 m/s. if the cue ball is at rest after the collision, how fast is the 8 ball traveling after the collision? 3) two football players are running toward each other. if the offensive player is 75 kg and is running 8 m/s, how fast must the 60 kg defensive player run in order for the two players to hit and stop?
Answers: 1

Chemistry, 22.06.2019 11:30
Compare and contrast refraction of light and sound will give brainliest
Answers: 1

Chemistry, 22.06.2019 12:00
What does a complete balanced chemical equation include? a. exothermic coefficients b. endothermic coefficients c. valence electrons d. molar coefficients
Answers: 1

Chemistry, 22.06.2019 22:30
The vapor pressure of ethanol is 1.00 × 102 mmhg at 34.90°c. what is its vapor pressure at 61.61°c? (δhvap for ethanol is 39.3 kj/mol.)
Answers: 2
You know the right answer?
Consider the following chemical reaction: 2KCl + 3O2 --> 2KClO3. If you are given 100.0 moles of...
Questions

Mathematics, 19.02.2021 23:20


Biology, 19.02.2021 23:20

Mathematics, 19.02.2021 23:20


Mathematics, 19.02.2021 23:20


Mathematics, 19.02.2021 23:20

Biology, 19.02.2021 23:20

Mathematics, 19.02.2021 23:20

English, 19.02.2021 23:20

Spanish, 19.02.2021 23:20


Social Studies, 19.02.2021 23:20

Mathematics, 19.02.2021 23:20




Mathematics, 19.02.2021 23:20

Mathematics, 19.02.2021 23:20