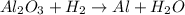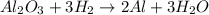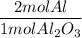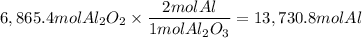Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 04:00
The rules of engagement (roe) working group is often used to (select all that apply.)
Answers: 2

Chemistry, 22.06.2019 08:30
If i initially have a gas at a pressure of 12 atm, a volume of 23 liters, and a temperature of 200 k, and then i raise the pressure to 14 atm and increase the temperature to 300 k, what is the new volume of the gas?
Answers: 1

Chemistry, 22.06.2019 14:50
Complete the following statements to describe solids, liquids, and gases. select the correct answer from each drop-down menu. a solid a definite volume and a definite shape. a liquid a definite volume and a definite shape. a gas a definite volume and a definite shape
Answers: 1

Chemistry, 22.06.2019 15:30
Which suspect most likely committed the robbery and how do you know
Answers: 2
You know the right answer?
A process to produce aluminum from aluminum oxide has an 85.0% yield. How much aluminum will be prod...
Questions

Mathematics, 24.03.2021 01:40

English, 24.03.2021 01:40

Geography, 24.03.2021 01:40

Mathematics, 24.03.2021 01:40





Mathematics, 24.03.2021 01:40


English, 24.03.2021 01:40

Mathematics, 24.03.2021 01:40

History, 24.03.2021 01:40


Mathematics, 24.03.2021 01:40

Mathematics, 24.03.2021 01:40


Mathematics, 24.03.2021 01:40