PLEASE ANSWER
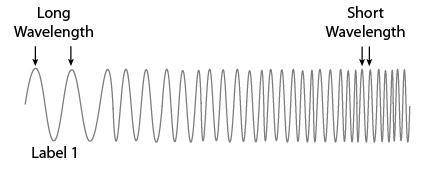
A student is making a model of the electromagnetic spectrum.
W...

Chemistry, 24.02.2020 02:50 htiffany0225
PLEASE ANSWER
A student is making a model of the electromagnetic spectrum.
Which text should the student use for label 1 to describe other properties of waves shown?
A. LOW FREQUENCY, LOW ENERGY
B. LOW FREQUENCY, HIGH ENERGY
C. HIGH FREQUENCY, LOW ENERGY
D. HIGH FREQUENCY, HIGH ENERGY

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 19:00
Nan element’s square on the periodic table, the number with the greatest numerical value represents the
Answers: 3

Chemistry, 22.06.2019 22:30
You just calculated that the heat of fusion for chloromethane is 6400 j/mol. the heat of fusion for hydrogen is 120 j/mol.? which of the following account for this difference? more than one correcta. chloromethane can absorb more energy at the same temperature. b. hydrogen has stronger intermolecular forces than chloromethane. c. hydrogen molecules can pack more closely than chloromethane molecules. d. chloromethane experiences dipole-dipole interactions. e. chloromethane has a higher molar mass than hydrogen.
Answers: 3

Chemistry, 22.06.2019 23:00
Which of your 24 wells had indications that a chemical reaction occurred? how were you able to tell that a chemical reaction occurred? which of your 24 wells had indications that a physical reaction occurred? how were you able to tell that a physical reaction occurred? report on both mixing and evaporation. make a general statement about whether your hypotheses were validated or rejected. must your hypotheses be correct for this to be a successful laboratory?
Answers: 3

Chemistry, 22.06.2019 23:10
Using the periodic table, complete the following. element: hydrogen symbol: h₂ molecular weight: g mass of one mole: g/mol
Answers: 3
You know the right answer?
Questions



Physics, 12.06.2021 23:00

Computers and Technology, 12.06.2021 23:00

Mathematics, 12.06.2021 23:00



Physics, 12.06.2021 23:00

Mathematics, 12.06.2021 23:00





Mathematics, 12.06.2021 23:00




Mathematics, 12.06.2021 23:10


English, 12.06.2021 23:10



