Chemistry, 23.02.2020 22:38 estherboocx
Determine the temperature (in K) of 0.6 moles of gas contained in a 1.7 L vessels at a pressure of 154.2 kPa.

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 18:00
Heat is the total potential energy of a substance that can be transferred. true false
Answers: 1

Chemistry, 23.06.2019 06:30
What is the chemical formula for a compound between li and br? libr li2br libr2 libr3
Answers: 1

Chemistry, 23.06.2019 14:00
[08.05] which answer provides the correct name for name the following hydrocarbon? (2 points) moving left to right: a hydrocarbon chain made of a methyl group (ch subscript three) single bond carbon triple bond carbon single bond ch bonded to a methyl group (ch subscript three) and a chain including a methelyne group (ch subscript two) single bond methyl group (ch subscript three). 4-ethyl-2-pentyne 4-methyl-2-hexyne 2-heptyne 4-methy-hexane
Answers: 1

Chemistry, 23.06.2019 18:30
What is the solution to the problem expressed to the correct number of significant figures? (102,900 ÷ 12) + (170 × 1.27) = ? a. 8,790 b. 8,790.9 c. 8,791 d. 8,800
Answers: 2
You know the right answer?
Determine the temperature (in K) of 0.6 moles of gas contained in a 1.7 L vessels at a pressure of 1...
Questions

Mathematics, 29.11.2021 17:30

Mathematics, 29.11.2021 17:30



Mathematics, 29.11.2021 17:30

Mathematics, 29.11.2021 17:30

Biology, 29.11.2021 17:30


English, 29.11.2021 17:30


Computers and Technology, 29.11.2021 17:30



Health, 29.11.2021 17:30

Mathematics, 29.11.2021 17:30

Mathematics, 29.11.2021 17:30



English, 29.11.2021 17:30

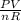
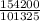 = 1.52atm
= 1.52atm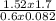 =
= 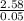 = 51.6K
= 51.6K