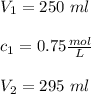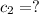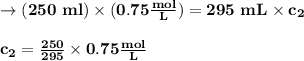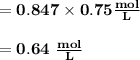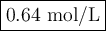Dilutions Worksheet - Solutions
If 45 mL of water are added to 250 mL of a 0.75 M K2SO4 soluti...

Chemistry, 23.02.2020 04:47 brainlord4209
Dilutions Worksheet - Solutions
If 45 mL of water are added to 250 mL of a 0.75 M K2SO4 solution, what will the
molarity of the diluted solution be?
(0.75 M)(250 ml) = M2 (295 mL)
M2 = (0.75 M) (250 mL) = 0.64 M
(295 mL)
Where did the 295ml came from

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 23:00
City a and city b had two different temperatures on a particular day. on that day, four times the temperature of city a was 8â° c more than 3 times the temperature of city b. the temperature of city a minus twice the temperature of city b was â’3â° c. what was the temperature of city a and city b on that day? city a was 5â° c, and city b was 4â° c. city a was 3â° c, and city b was â’1â° c. city a was 8â° c, and city b was â’3â° c. city a was 5â° c, and city b was â’5â° c.
Answers: 2

Chemistry, 22.06.2019 07:00
How many moles are in 7.2 x 10^23 carbon molecules? (*round to the nearest hundredth and include the unit "mol c" after your number) question 6 options:
Answers: 2

Chemistry, 22.06.2019 16:00
Which of the following is the correct definition of chemical energy? a. energy an object has because of its motion or position b. energy resulting from the flow of charged particles, such as electrons or ions c. energy produced from the splitting of atoms d. energy stored in chemical bonds of molecules
Answers: 1

You know the right answer?
Questions

Mathematics, 19.08.2019 11:00

French, 19.08.2019 11:00

Mathematics, 19.08.2019 11:00



English, 19.08.2019 11:00

Mathematics, 19.08.2019 11:00

Mathematics, 19.08.2019 11:00



English, 19.08.2019 11:00


Chemistry, 19.08.2019 11:00


History, 19.08.2019 11:00

Physics, 19.08.2019 11:00


English, 19.08.2019 11:00