Chemistry, 23.02.2020 00:22 JamierW2005
Using the symbol below/above determine the number of protons, neutrons, and electrons in the element.
A. Protons = 50
Neutrons = 118
Protons = 46
B. Protons = 68
Neutrons = 46
Electrons = 54
C. Protons = 50
Neutrons = 68
Electrons = 46
D. Protons = 118
Neutrons = 50
Electrons = 46
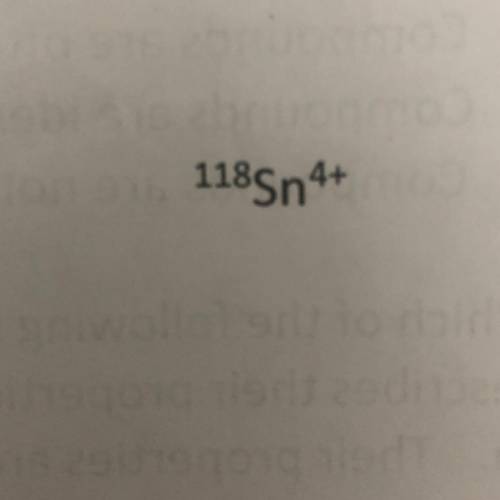

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 12:00
In the following redox reaction which is the oxidizing agent and which is the reducing agent? alcl3 + na nacl + al oxidizing agent = reducing agent =
Answers: 1

Chemistry, 22.06.2019 15:30
Two metal blocks that have slightly different temperatures are placed next to one another. after five minutes, they both have lower but equal temperatures. according to the law of conservation of energy, what most likelyhappened? energy was created inside the blocks.energy was destroyed inside the blocks.energy was absorbed into the blocks from outside the system.energy was transferred from the warmer block to the cooler block.
Answers: 2

Chemistry, 22.06.2019 21:00
Two nails have identical sizes and shapes. in one nail, 20 percent of the domains are lined up. in the other nail, 80 percent of the domains are lined up. which has stronger magnetic force? first answer gets brainliest!
Answers: 1

Chemistry, 23.06.2019 00:20
Which diagram represents the phase tha occurs after a solid melts?
Answers: 1
You know the right answer?
Using the symbol below/above determine the number of protons, neutrons, and electrons in the element...
Questions


Computers and Technology, 25.03.2021 16:50


Mathematics, 25.03.2021 16:50

Mathematics, 25.03.2021 16:50

History, 25.03.2021 16:50

Mathematics, 25.03.2021 16:50

History, 25.03.2021 16:50



Mathematics, 25.03.2021 16:50

Mathematics, 25.03.2021 16:50


Mathematics, 25.03.2021 16:50

Mathematics, 25.03.2021 16:50

History, 25.03.2021 16:50

Mathematics, 25.03.2021 16:50

Social Studies, 25.03.2021 16:50


Geography, 25.03.2021 16:50