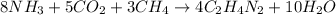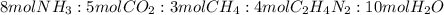Chemistry, 22.02.2020 22:20 Scourge927
One of the intermediates in the synthesis of glycine from ammonia, carbon dioxide, and methane is aminoacetonitrile, C2H4N2.
How much C2H4N2 could be expected from the reaction of 14.9 g CO2, 2.11 g NH3, and 1.75 g CH4?

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 22:30
Write the symbol for every chemical element that has atomic number greater than 3 and atomic mass less than 12.0 u.
Answers: 1

Chemistry, 22.06.2019 14:30
According to le chatelier’s principle, a system in chemical equilibrium responds to stress by shifting the equilibrium in a direction that reduces the stress. normalizes the stress. increases the stress. changes the stress.
Answers: 1

Chemistry, 22.06.2019 18:50
Which of the following is a conclusion that resulted from ernest rutherford’s scattering experiment? (will mark brainliest) a. the nucleus is negatively charged b. the atom is a dense solid and is indivisible c. the mass is conserved when atoms react chemically d. the nucleus is very small and the atom is mostly empty space
Answers: 3

Chemistry, 22.06.2019 21:30
Isopropyl alcohol, (ch3)2choh, is a common solvent. determine the percent by mass of hydrogen in isopropyl alcohol. a) 6.71% h b) 13.4% h c) 25.0% h d) 53.3% h
Answers: 1
You know the right answer?
One of the intermediates in the synthesis of glycine from ammonia, carbon dioxide, and methane is am...
Questions



Physics, 05.01.2020 12:31

Mathematics, 05.01.2020 12:31


Mathematics, 05.01.2020 12:31

Mathematics, 05.01.2020 12:31


History, 05.01.2020 12:31


Social Studies, 05.01.2020 12:31



History, 05.01.2020 12:31


History, 05.01.2020 12:31

Mathematics, 05.01.2020 12:31

Mathematics, 05.01.2020 12:31