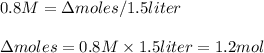Chemistry, 22.02.2020 18:57 kaleahearly123
Consider the following reaction:
2H2O2(aq)→2H2O(l)+O2(g)
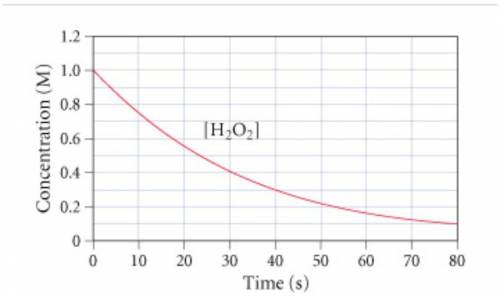
The graph (Figure 1) shows the concentration of H2O2 as a function of time.
If the instantaneous rate of formation of O2 is 3.3*(10^-3) moles/(liters*seconds), then...
If the initial volume of the H2O2 solution is 1.5 L , what total amount of O2 (in moles) is formed in the first 50 s of reaction?
Express your answer using two significant figures.

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 15:30
The reactions of photosynthesis occur in the of plant cell? a.mitochondria. b. lysosomes. c. chloroplasts. d. chlorophyll
Answers: 1

Chemistry, 22.06.2019 21:00
In the experiment you asked to react hydrochloric acid and with sodium hydroxide. when measuring the volume of the reactants, which instrument would give the greatest precision.
Answers: 3


Chemistry, 23.06.2019 00:30
In a ball-and-stick molecular model, what do the sticks represent?
Answers: 1
You know the right answer?
Consider the following reaction:
2H2O2(aq)→2H2O(l)+O2(g)
The graph (Figure 1...
2H2O2(aq)→2H2O(l)+O2(g)
The graph (Figure 1...
Questions




Geography, 23.12.2019 18:31



Social Studies, 23.12.2019 18:31




Computers and Technology, 23.12.2019 18:31







Computers and Technology, 23.12.2019 18:31

Computers and Technology, 23.12.2019 18:31

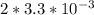
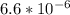
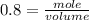
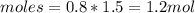
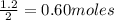 O₂ is formed in the first 50 s of reaction.
O₂ is formed in the first 50 s of reaction.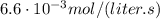
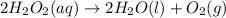

![\Delta [H_2O_2]=\Delta moles/Volume(liters)](/tpl/images/0520/3300/3d5f9.png)