
Chemistry, 22.02.2020 09:38 XxKaitlynnxX
A process to produce aluminum from aluminum oxide has an 85.0% yield. How much aluminum will be produced from a reaction 700.0 kg of aluminum oxide to produce Al? (assume that the reaction is Al2O3 + H2=Al+H2O

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 17:00
How can a give a full method for the experiment of separating sand from water by filtration? 1-materials 2-steps 3-conclusion also for water and salt separated by the evaporation or distillation process
Answers: 1

Chemistry, 22.06.2019 17:10
In which block of the periodic table is uranium (u) found? s blockd blockp blockf block
Answers: 1


You know the right answer?
A process to produce aluminum from aluminum oxide has an 85.0% yield. How much aluminum will be prod...
Questions


Chemistry, 17.02.2021 23:20

Mathematics, 17.02.2021 23:20



Mathematics, 17.02.2021 23:20

Mathematics, 17.02.2021 23:20

Biology, 17.02.2021 23:20









Chemistry, 17.02.2021 23:20

Mathematics, 17.02.2021 23:20


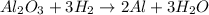
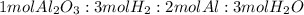
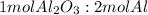 ; thus 6,865.4 mol of aluminium oxide produce 2 × 6,865.4 mol of aluminum = 13,730.8 mol of aluminium.
; thus 6,865.4 mol of aluminium oxide produce 2 × 6,865.4 mol of aluminum = 13,730.8 mol of aluminium.

