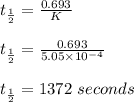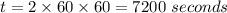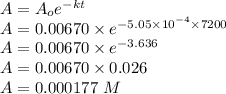Chemistry, 22.02.2020 04:59 francisebell2p698f2
Consider the first-order reaction described by the equation Cyclopropane gas isomerizes to propene gas. At a certain temperature, the rate constant for this reaction is 5.05 × 10 − 4 s − 1 . Calculate the half-life of cyclopropane at this temperature. t 1 / 2 = s Given an initial cyclopropane concentration of 0.00670 M , calculate the concentration of cyclopropane that remains after 2.00 hours. concentration.

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 03:30
Calculate the molar mass of aluminum oxide (al2o3). express your answer to four significant figures.
Answers: 1

Chemistry, 22.06.2019 05:30
Describe the interaction that occurs between two objects with the same electrical charge.
Answers: 1


Chemistry, 23.06.2019 00:00
The empirical formula of a compound is ch2o and its mass is 120 amu/molecule, what is its formula?
Answers: 2
You know the right answer?
Consider the first-order reaction described by the equation Cyclopropane gas isomerizes to propene g...
Questions

English, 17.10.2021 23:00

Mathematics, 17.10.2021 23:00


Mathematics, 17.10.2021 23:00


Biology, 17.10.2021 23:00


History, 17.10.2021 23:00

Mathematics, 17.10.2021 23:00





Mathematics, 17.10.2021 23:00


Business, 17.10.2021 23:00

Mathematics, 17.10.2021 23:00

Mathematics, 17.10.2021 23:00

Mathematics, 17.10.2021 23:00

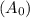 of was
of was 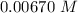 .
.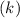 is
is 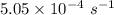
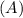 after 2 hours.
after 2 hours.