
Chemistry, 22.02.2020 03:32 bibigardeax
Mastering chem If the experiment below is run for 60 s, 0.16 mol A remain. Which of the following statements is or are true? At time 0 seconds, a flask contains 1.00 mole of A and 0 moles of B. At 20 seconds, the flask holds 0.54 moles of A and 0.46 moles of B. At 40 seconds, the flask holds 0.3 moles of A and 0.7 moles of B. After 60 s there are 0.84 mol B in the flask. The decrease in the number of moles of A from t1 = 0 to t2 = 20 s is greater than that for t1 = 40 to t2 = 60 s. The average rate for the reaction from t1 = 40 to t2 = 60 s is 7.0×10−3 M/s.

Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 18:40
Determine the energy released per kilogram of fuel used. given mev per reaction, calculate energy in joules per kilogram of reactants. consider 1 mole of tritium plus 1 mole of deuterium to be a mole of “reactions” (total molar mass = 5 grams).
Answers: 1

Chemistry, 22.06.2019 03:00
Select all that apply. a beta particle: is electromagnetic energy is an electron has zero charge is emitted from the nucleus has a +2 charge has a -1 charge
Answers: 1

Chemistry, 22.06.2019 06:30
Type the correct answer in the box. spell all words correctly.what is the correct term for living the most sustainable life you can within your current circumstances? when your are being as sustainable as you can within your current lifestyle, you are said to be sustainability.
Answers: 3

Chemistry, 22.06.2019 17:00
Reduction is a reaction which results in a in electrons and a in positive charge of the atom or ion 1) a- loss 1) b- gain 2) a-increase 2) b-decrease
Answers: 1
You know the right answer?
Mastering chem If the experiment below is run for 60 s, 0.16 mol A remain. Which of the following st...
Questions



Mathematics, 02.04.2020 23:59


Spanish, 02.04.2020 23:59

History, 02.04.2020 23:59


Mathematics, 02.04.2020 23:59

Mathematics, 02.04.2020 23:59

Social Studies, 02.04.2020 23:59

Mathematics, 02.04.2020 23:59





Social Studies, 02.04.2020 23:59



Mathematics, 02.04.2020 23:59

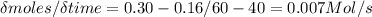 which is true as well
which is true as well 


