Chemistry, 22.02.2020 02:58 borgesalfonso12
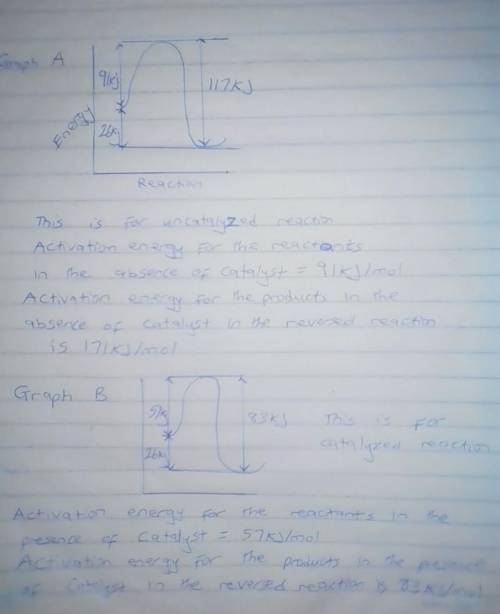
A catalyst decreases the activation energy of a particular exothermic reaction by 34 kJ/mol, to 57 kJ/mol. Assuming that the mechanism has only one step, and that the products are 26 kJ lower in energy than the reactants, sketch approximate energy-level diagrams for the catalyzed and uncatalyzed reactions. What is the activation energy for the uncatalyzed reverse reaction?

Answers: 1


Another question on Chemistry


Chemistry, 22.06.2019 03:30
What is the number of moles of chemical units represented by 9.03x10^24? and how do i show work? (dumb it down )
Answers: 1


You know the right answer?
A catalyst decreases the activation energy of a particular exothermic reaction by 34 kJ/mol, to 57 k...
Questions



Mathematics, 23.09.2019 06:10

Mathematics, 23.09.2019 06:10

Business, 23.09.2019 06:10


English, 23.09.2019 06:10

Mathematics, 23.09.2019 06:10

Biology, 23.09.2019 06:10




Social Studies, 23.09.2019 06:10




Mathematics, 23.09.2019 06:10



History, 23.09.2019 06:10