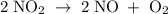Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 10:20
Gwhich r group would most likely be found in a hydrophobic area of the tertiary structure of a globular protein? which r group would most likely be found in a hydrophobic area of the tertiary structure of a globular protein? −ch2−oh −ch2−o||c−nh2 −ch2−coo− −ch2−ch2−ch2−ch2−n+h3
Answers: 3

Chemistry, 23.06.2019 07:10
1) a light bulb takes in 30 of energy per second. it transfers 3j as use energy. calculate the efficiency. second. it transfers 3j as useful light energy and 27j as heat energy. calculate the efficiency
Answers: 1


Chemistry, 23.06.2019 12:00
372 ml is the volume of aluminum, density is 2.70 g/ml what is the mass in grams
Answers: 1
You know the right answer?
If one starts with pure NO2(g) at a pressure of 0.500 atm, the total pressure inside the reaction ve...
Questions


Geography, 11.05.2021 05:30

Computers and Technology, 11.05.2021 05:30





Physics, 11.05.2021 05:30

Mathematics, 11.05.2021 05:30

Mathematics, 11.05.2021 05:30







Mathematics, 11.05.2021 05:30

Mathematics, 11.05.2021 05:30

Mathematics, 11.05.2021 05:30

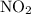 at equilibrium has been 0.152 atm.
at equilibrium has been 0.152 atm.