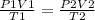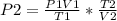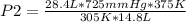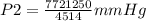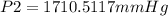Chemistry, 22.02.2020 02:28 jaksmmwlqlzm
A sample of gas with an initial volume of 28.4 Liters at a pressure of 725 mmHg and a temperature of 305 K is compressed to a volume of 14.8 Liters and warmed to a temperature of 375 Kelvin. What is the final pressure of the gas?

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 08:30
For each of the compounds below, show that the charges on the ions add up to zero. a. kbr b. cao c. li(2)o d. cacl(2) e. alcl(3)
Answers: 2


Chemistry, 22.06.2019 12:00
Ican determine the molar mass of an element by looking on the under the atomic mass for the element. for example the molar mass of phosphorus is 30.974 grams/mole. avogadro’s number tells me the amount of representative particles in 1 mole of any substance. this means 12.011 gram sample of carbon and a 32.0 gram sample of sulfur have the same number of atoms.
Answers: 1

Chemistry, 22.06.2019 18:00
Heat is the total potential energy of a substance that can be transferred. true false
Answers: 1
You know the right answer?
A sample of gas with an initial volume of 28.4 Liters at a pressure of 725 mmHg and a temperature of...
Questions

Mathematics, 25.02.2020 03:02

Mathematics, 25.02.2020 03:02

Computers and Technology, 25.02.2020 03:02
















Mathematics, 25.02.2020 03:02

English, 25.02.2020 03:02