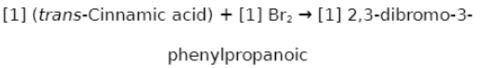Chemistry, 22.02.2020 02:06 jennyferluna0216
Suppose a student started with 142.0 mg of trans-cinnamic acid, 412 mg of pyridinium tribromide, and 2.30 mL of glacial acetic acid. After the reaction and workup, the student ended up with 0.2223 g of brominated product. Calculate the student\'s theoretical and percent yields.

Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 17:10
There are 6.022 x 10^23 atoms of hg in 1 mole of hg. the number of atoms in 4.5 moles of hg can be found by multiplying 4.5 by 6.022 x 10^23 a. 2.7 x 10^24 b. 27 x 10^23 c. 2.71 x10^24 d. 27.099 x 10^23
Answers: 3

Chemistry, 21.06.2019 21:40
It is important to wear proper protective equipment in lab even when not actively performing experiments because accidents can affect any researcher, even one not working on an experiment. select the best answer from the choices provided
Answers: 3

Chemistry, 21.06.2019 22:30
This large tectonic plate is bounded on three sides by whats know as the ring of fire. what is the name of this tectonic plate? a) pacific plate b) eurasian plate c) north american plate d) indo- australian plate plz it's science but there's no option for science so i picked chemistry
Answers: 2

You know the right answer?
Suppose a student started with 142.0 mg of trans-cinnamic acid, 412 mg of pyridinium tribromide, and...
Questions

Geography, 04.09.2020 05:01


Mathematics, 04.09.2020 05:01





Mathematics, 04.09.2020 05:01











History, 04.09.2020 05:01

Mathematics, 04.09.2020 05:01