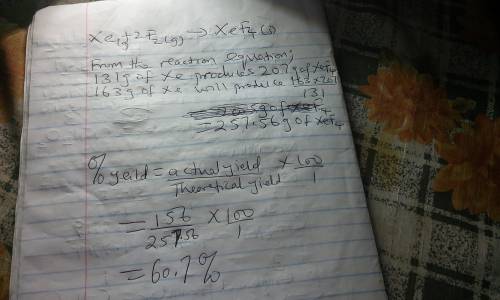Chemistry, 22.02.2020 01:45 cjtambasco
Although they were formerly called the inert gases, at least the heavier elements of Group 8 do form relatively stable compounds. For example, xenon combines directly with elemental fluorine at elevated temperatures in the presence of a nickel catalyst. Use table 1 and table 2. Xe(g) + 2 F2(g) → XeF4(s) What is the theoretical mass of xenon tetrafluoride that should form when 163 g of xenon is reacted with 164 g of F2? g= What is the percent yield if only 156 g of XeF4 is actually isolated?

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 00:30
This is a characteristic of the elements in the periodic table that shows a pattern. it may increase or decrease across or down the table.
Answers: 1


Chemistry, 22.06.2019 04:50
Compare the equilibrium constants for the systems shown in the table. which favors products the most? which favors products the least? rank these systems in order from most to least in terms of favoring products rather than reactants. d > b > a > c c > a > b > d b > c > d > a a > d > c > b
Answers: 1

Chemistry, 22.06.2019 05:30
Which other elements contain the same number of outer electrons as sodium
Answers: 3
You know the right answer?
Although they were formerly called the inert gases, at least the heavier elements of Group 8 do form...
Questions

Mathematics, 29.06.2019 19:00


Mathematics, 29.06.2019 19:00

Mathematics, 29.06.2019 19:00

English, 29.06.2019 19:00


Mathematics, 29.06.2019 19:00

Mathematics, 29.06.2019 19:00

Geography, 29.06.2019 19:00





Mathematics, 29.06.2019 19:00

Biology, 29.06.2019 19:00




Computers and Technology, 29.06.2019 19:00