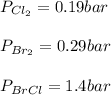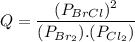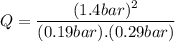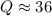Chemistry, 21.02.2020 23:49 Katlynnmarkle13
Consider the gas-phase reaction, Cl2(g) + Br2(g) <=> 2 BrCl(g), for which Kp = 32 at 500 K. If the mixture is analyzed and found to contain 0.19 bar of Cl2, 0.29 bar of Br2 and 1.4 bar of BrCl, describe the situation:a) Q > K and more reactants will be made to reach equilibrium. b) Q > K and more products will be made to reach equilibrium. c) Within 1 decimal place, Q = K and the reaction is at equilibriumd) Q < K and more products will be made to reach equilibrium. e) Q < K and more reactants will be made to reach equilibrium.

Answers: 2


Another question on Chemistry


Chemistry, 22.06.2019 13:30
Apush or pull that moves or changes and object when to objects touch
Answers: 2

Chemistry, 23.06.2019 13:30
The zinc within a copper-plated penny dissolves in hydrochloric acid if the copper coating is filed down in several spots (so that the hydrochloric acid can reach the zinc). the reaction between the acid and the zinc 2h+(aq)+zn(s)→h2(g)+zn2+(aq) . when the zinc in a certain penny dissolves, the total volume of gas collected over water at 25 °c is 0.947 l at a total pressure of 743 mmhg . (vapor pressure of water is 23.78 mmhg at 25 °c .) what mass of hydrogen gas is collected? answer in appropriate significant figures
Answers: 3

Chemistry, 23.06.2019 15:30
The gas in a sealed container has an absolute pressure of 9.25 atmospheres. if the air around the container is at standard pressure, what is the gauge pressure inside the container
Answers: 1
You know the right answer?
Consider the gas-phase reaction, Cl2(g) + Br2(g) <=> 2 BrCl(g), for which Kp = 32 at 500 K. If...
Questions

History, 18.05.2021 06:20

Chemistry, 18.05.2021 06:20

History, 18.05.2021 06:20

Mathematics, 18.05.2021 06:20

English, 18.05.2021 06:20



Mathematics, 18.05.2021 06:20

Mathematics, 18.05.2021 06:20

Chemistry, 18.05.2021 06:20


History, 18.05.2021 06:20


Mathematics, 18.05.2021 06:20


Mathematics, 18.05.2021 06:20




Mathematics, 18.05.2021 06:20