
Chemistry, 21.02.2020 23:28 kayleegeise
The Henry's Law constant (kH) for carbon monoxide in water at 25°C is 9.71×10-4 mol/L·atm. How many grams of CO will dissolve in 1.00 L of water if the partial pressure of CO is 2.75 atm?

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 15:20
Draw any one of the skeletal structures of a 2° alkyl bromide having the molecular formula of c6h13br and two stereogenic centers. indicate chirality by using wedge and hashed wedge notation. lone pairs do not need to be shown.
Answers: 1

Chemistry, 22.06.2019 18:30
The number of moles of a given mass of a substance can be found without knowing its molecular formula or molar mass. true false
Answers: 1

Chemistry, 22.06.2019 22:00
If a solution contains 3 moles/liter of sodium chloride (nacl, made of sodium ions and chloride ions), what is the osmolarity of this solution
Answers: 3

Chemistry, 23.06.2019 00:30
Gasoline has a density of 0.740 g/ml. if you have 328 grams of gasoline, what is the volume in milliliters?
Answers: 1
You know the right answer?
The Henry's Law constant (kH) for carbon monoxide in water at 25°C is 9.71×10-4 mol/L·atm. How many...
Questions




Mathematics, 03.12.2021 18:50

Mathematics, 03.12.2021 18:50

English, 03.12.2021 18:50


Physics, 03.12.2021 18:50


English, 03.12.2021 18:50



Chemistry, 03.12.2021 18:50




Chemistry, 03.12.2021 18:50

Mathematics, 03.12.2021 18:50

Mathematics, 03.12.2021 18:50


 = Henry's constant =
= Henry's constant = 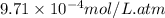
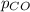 = partial pressure of CO = 2.75 atm
= partial pressure of CO = 2.75 atm




