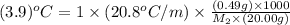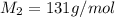Chemistry, 21.02.2020 22:44 princessammarah4731
When 0.49 g of a molecular compound was dissolved in 20.00 g of cyclohexane, the freezing point of the solution was lowered by 3.9 0C. Determine the molecular mass of this compound.

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 21:40
It is important to wear proper protective equipment in lab even when not actively performing experiments because accidents can affect any researcher, even one not working on an experiment. select the best answer from the choices provided
Answers: 3

Chemistry, 22.06.2019 10:10
Stage in which a typical star has completely stopped fusion
Answers: 1

Chemistry, 22.06.2019 12:10
Achemistry student needs to standardize a fresh solution of sodium hydroxide. he carefully weighs out of oxalic acid , a diprotic acid that can be purchased inexpensively in high purity, and dissolves it in of distilled water. the student then titrates the oxalic acid solution with his sodium hydroxide solution. when the titration reaches the equivalence point, the student finds he has used of sodium hydroxide solution.calculate the molarity of the student's sodium hydroxide solution. be sure your answer has the correct number of significant digits.
Answers: 1

Chemistry, 22.06.2019 14:00
What is the ph of a solution that has a hydrogen ion concentration of 1.0 * 10 -9 m?
Answers: 2
You know the right answer?
When 0.49 g of a molecular compound was dissolved in 20.00 g of cyclohexane, the freezing point of t...
Questions

Mathematics, 09.04.2021 16:40

Mathematics, 09.04.2021 16:40

Geography, 09.04.2021 16:40

Mathematics, 09.04.2021 16:40


Social Studies, 09.04.2021 16:40


Biology, 09.04.2021 16:40


Biology, 09.04.2021 16:40

Mathematics, 09.04.2021 16:40

Mathematics, 09.04.2021 16:40

Mathematics, 09.04.2021 16:40







Mathematics, 09.04.2021 16:40

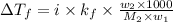
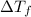 = depression in freezing point =
= depression in freezing point = 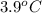
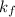 = freezing point constant =
= freezing point constant = 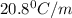
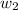 = mass of solute = 0.49 g
= mass of solute = 0.49 g
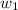 = mass of solvent (cyclohexane) = 20.00 g
= mass of solvent (cyclohexane) = 20.00 g
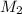 = molar mass of solute = ?
= molar mass of solute = ?