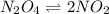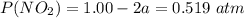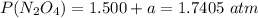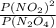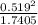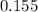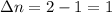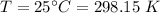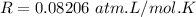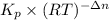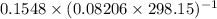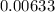Chemistry, 21.02.2020 22:19 hartzpeyton136
A flask is charged with 1.500atm of N2O4(g) and 1.00 atm NO2(g) at 25 degree C, and the following equilibrium is achieved: N2O4(g) 2NO2 After equilibrium is reached, the partial pressure of NO2 is 0.519atm. Calculate the value of Kp for the reaction. Calculate Kc for the reaction.

Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 16:30
Find the empirical formula of each of the following compounds. given mass or for each element in a sample of the compound 3,611 g ca; 6.389 g c1
Answers: 1

Chemistry, 21.06.2019 23:30
What’s the scientific notation for the number 6,840,000,000
Answers: 1


Chemistry, 22.06.2019 08:00
Why is the bond angle in a water molecule less than the bond angle of methane? a. the central oxygen atom in water has two lone pairs of electrons, whereas the central carbon atom in methane has no lone pairs. b. the central hydrogen atom in water has one lone pair of electrons, whereas the central carbon atom in methane has two lone pairs. c. the central oxygen atom in water has four lone pairs of electrons, whereas the central carbon atom in methane has only one lone pair. d. the central oxygen atom exerts more repulsive force on surrounding atoms than the central carbon atom in methane does. reset next
Answers: 2
You know the right answer?
A flask is charged with 1.500atm of N2O4(g) and 1.00 atm NO2(g) at 25 degree C, and the following eq...
Questions

Mathematics, 26.09.2019 15:50




Social Studies, 26.09.2019 15:50

Physics, 26.09.2019 15:50

Health, 26.09.2019 15:50

Chemistry, 26.09.2019 15:50


English, 26.09.2019 15:50

Mathematics, 26.09.2019 15:50


History, 26.09.2019 15:50



English, 26.09.2019 15:50

Physics, 26.09.2019 15:50

Health, 26.09.2019 15:50

Mathematics, 26.09.2019 15:50