Chemistry, 21.02.2020 21:53 jalenshayewilliams
5. The gas-phase decomposition of ethyl iodide to give ethylene and hydrogen iodide is a first-order reaction. C2H5I C2H4 + HI At 600 K, the value of k is 1.60 × 10– 5 s– 1. When the temperature is raised to 700 K, the value of k increases to 6.36 × 10– 3 s– 1. What is the activation energy for this reaction?

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 12:50
What is the chemical name of the compound na2co3? use the list of polyatomic ions and the periodic table to you answer. a. sodium carbon oxide b. sodium carbonate c. sodium(ll) carbonate d. sodium oxalate
Answers: 1

Chemistry, 22.06.2019 20:00
I’m an electrically neutral atomic any element, there are equal numbers of
Answers: 2

Chemistry, 22.06.2019 23:00
What is the most common reason for matter changing its state?
Answers: 1

Chemistry, 23.06.2019 05:30
Based on the formulas, select the compounds below that are covalent: kbr sif4 al2o3 co2 naco3 s7o2 pcl3 fe3n2 h2o s2f10
Answers: 3
You know the right answer?
5. The gas-phase decomposition of ethyl iodide to give ethylene and hydrogen iodide is a first-order...
Questions



Mathematics, 12.03.2020 06:03













Mathematics, 12.03.2020 06:03



Mathematics, 12.03.2020 06:03

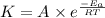
![\log (\frac{K_2}{K_1})=\frac{E_a}{2.303\times R}[\frac{1}{T_1}-\frac{1}{T_2}]](/tpl/images/0519/6420/1504e.png)
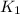 = rate constant at
= rate constant at 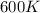 =
= 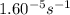
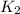 = rate constant at
= rate constant at 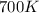 =
= 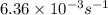
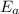 = activation energy for the reaction = ?
= activation energy for the reaction = ?
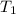 = initial temperature =
= initial temperature = 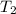 = final temperature =
= final temperature = ![\log (\frac{6.36\times 10^{-3}s^{-1}}{1.60\times 10^{-5}s^{-1}})=\frac{E_a}{2.303\times 8.314J/mole.K}[\frac{1}{600K}-\frac{1}{700K}]](/tpl/images/0519/6420/54c04.png)
![2.60=\frac{E_a}{2.303\times 8.314J/mole.K}[\frac{1}{600K}-\frac{1}{700K}]](/tpl/images/0519/6420/52269.png)